When your pacemaker fails during surgery or your MRI machine glitches mid-scan, lives hang in the balance. Medical PCBs power devices we bet our lives on daily – but what makes them different from regular circuit boards?
Medical PCBs are specialized printed circuit boards designed for healthcare applications. They prioritize reliability, precision, and compliance with strict medical safety standards to ensure error-free operation in life-critical equipment like defibrillators and patient monitors.
The stakes in medical electronics dwarf consumer gadget requirements. As we dissect four key aspects of medical PCBs, you’ll discover why your smartphone’s circuit board could never power an ICU ventilator – and what makes medical-grade electronics uniquely demanding.
How Are Medical PCBs Different from Industrial/Consumer PCBs?
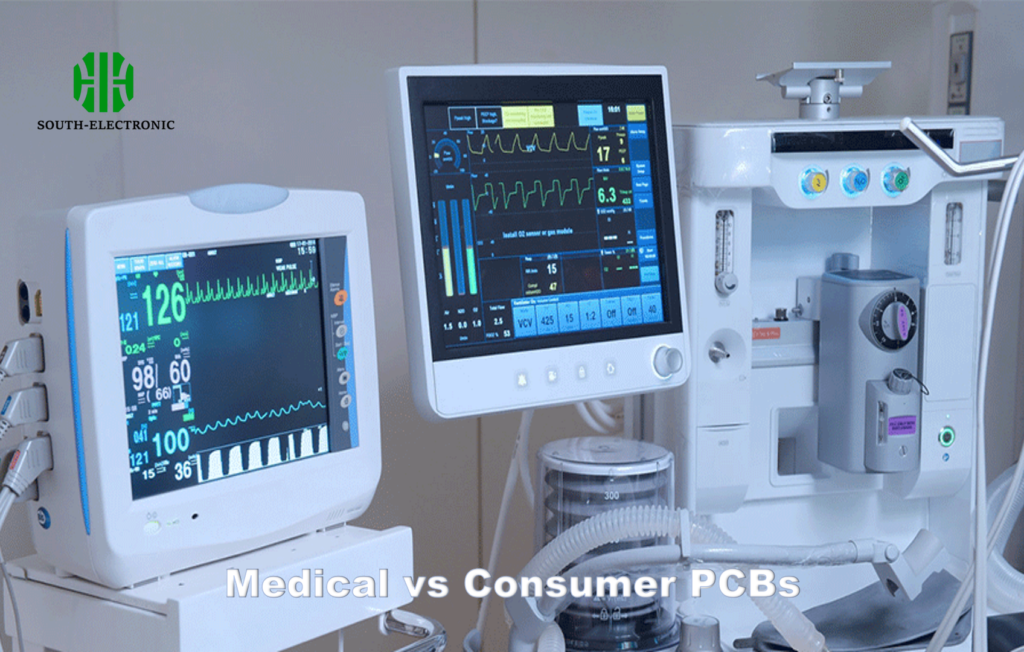
A single capacitor failure shut down your TV. The same component failure in an infusion pump could kill someone. Medical PCBs face unique operational demands that consumer electronics never encounter.
Medical PCBs[^1] require military-grade reliability[^2], sterilization compatibility[^3], and failsafe redundancy systems absent in commercial boards. They undergo 10× more rigorous testing cycles while maintaining precision in hostile clinical environments.
)
Three Critical Differentiators
-
Failure Consequences
Consumer PCB flaws cause inconveniences. Medical PCB errors create life-threatening risks:Application Consumer Impact Medical Impact Component Failure Device restart Patient death Signal Noise Audio crackle Misdiagnosis Latency Issues Video lag Delayed defibrillation -
Environmental Challenges
Medical PCBs withstand:- Repeated chemical sterilization
- Body fluid exposure
- Electrosurgical interference
- Extreme temperature cycles (-20°C to 50°C)
-
Longevity Requirements
Industrial PCBs last 5-7 years. Implanted medical PCBs like pacemakers must operate flawlessly for 10+ years without physical maintenance.
What Standards Must Medical PCBs Comply With? (IPC, ISO 13485, FDA)
Would you board a plane without FAA certification? Medical PCBs undergo stricter compliance checks than aviation electronics before touching human lives.
Medical PCBs must meet IPC-A-610 Class 3[^4] standards, ISO 13485[^5] quality systems, and FDA 21 CFR Part 820[^6] regulations. These enforce traceable manufacturing, biocompatibility testing, and risk management protocols exceeding aerospace requirements.
)
The Compliance Trinity Explained
-
IPC-A-610 Class 3
Defines acceptance criteria for high-reliability electronics:Parameter Class 2 (Automotive) Class 3 (Medical) Solder Gap 50% pad coverage 75% pad coverage Cleanliness 1.56 μg/cm² NaCl 0.1 μg/cm² NaCl Testing Sample basis 100% inspection -
ISO 13485
Mandates:- Full component traceability
- Sterilization validation
- Biocompatibility documentation
- Post-market surveillance
-
FDA 21 CFR Part 820
Requires:- Design controls (DHF)
- Device Master Record (DMR)
- Corrective Action Plans (CAPA)
- Installation qualification (IQ/OQ/PQ)
Non-compliance risks device recalls averaging $600M and criminal charges under FD&C Act Section 301.
Which Medical Applications Rely Most Heavily on Advanced PCBs?
When radiologists detected my uncle’s tumor early, they weren’t just reading scans – they were interpreting data from millions of PCB-connected sensors. Modern medicine literally runs on circuit boards.
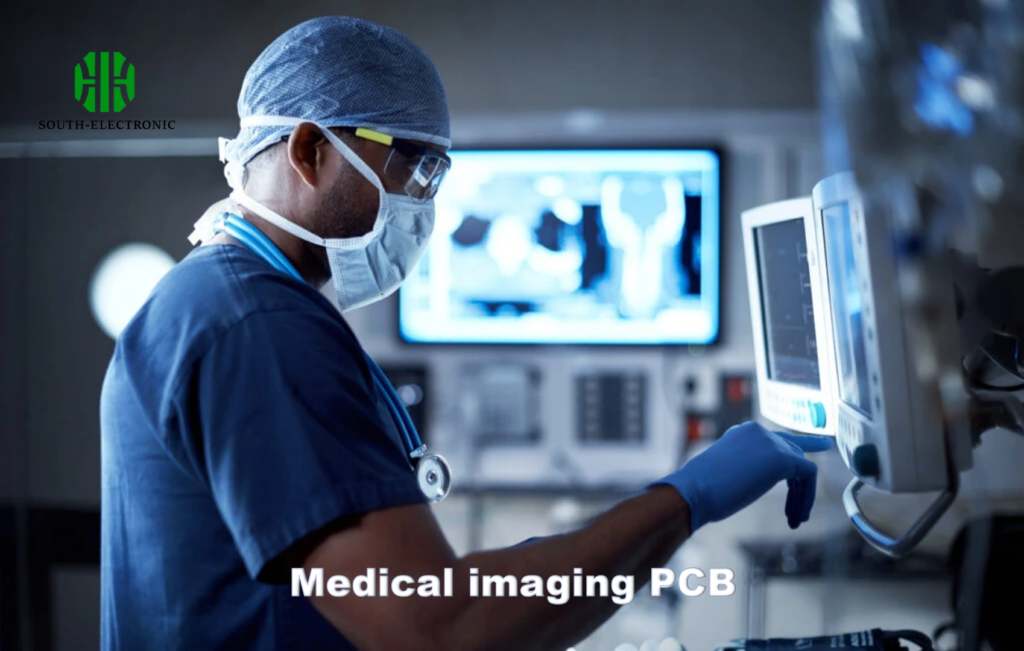
High-reliance medical PCB applications[^7] include diagnostic imaging[^8] (CT/MRI), patient monitoring systems, surgical robots, and implantables like neurostimulators. These use HDI PCBs with 20+ layers and 0.1mm microvias for signal integrity.
)
PCB-Dependent Medical Technologies
-
Diagnostic Imaging
- CT scanners: 150kW PCBs handling 4000+ simultaneous slices
- MRI machines: RF shielding for 3T magnetic fields
- Ultrasound: Analog/digital hybrid boards
-
Life Support Systems
- Ventilators: Redundant oxygen sensor PCBs
- Dialysis: Fluidics control boards
- ECMO: Blood pump motor controllers
-
Minimally Invasive Surgery
- Endoscopes: Camera & LED driver boards
- Surgical robots: MIL-SPEC flex PCBs
- Laser systems: Thermal management boards
Advanced PCBs enabled 87% reduction in diagnostic errors since 2010 according to JAMA studies.
Why Is Miniaturization Critical for Medical PCBs Today?
The pacemaker in my neighbor’s chest is smaller than a matchbox – yet contains 8 PCB layers monitoring 15 cardiac parameters. Miniaturization revolutionizes patient care through less invasive procedures.
PCB miniaturization[^9] allows swallowable endoscopy capsules, subcutaneous glucose monitors, and targeted drug delivery microchips. Shrinking components enable 60% smaller implants while increasing functionality through embedded AI processors.
)
The Shrinkage Paradox
-
Technical Challenges
- Managing heat in 0.4mm PCBs
- 01005 component placement (0.4×0.2mm)
- Microvia filling (<50μm holes)
- Flex-rigid board integration
-
Patient Benefits
- 70% less surgical scarring
- Implant durations up to 15 years
- Real-time analytics via onboard ML
-
Material Innovations
- Liquid crystal polymer (LCP) substrates
- Low-loss PTFE dielectrics
- Bioabsorbable magnesium circuits
Miniaturized PCBs reduced average hospital stays by 3.2 days (CDC 2023), saving $12B annually in US healthcare costs.
Conclusion
Medical PCBs form the silent backbone of modern healthcare, combining extreme reliability with cutting-edge miniaturization to enable life-saving technologies while meeting humanity's highest safety standards.
[^1]: Explore this link to understand the critical role of Medical PCBs in healthcare and their unique requirements for safety and reliability.
[^2]: Discover the importance of military-grade reliability in Medical PCBs and how it ensures patient safety in critical situations.
[^3]: Learn why sterilization compatibility is essential for Medical PCBs to function safely in clinical environments and protect patient health.
[^4]: Understanding IPC-A-610 Class 3 standards is crucial for ensuring high-reliability in medical electronics. Explore this link for detailed insights.
[^5]: ISO 13485 is essential for quality management in medical devices. Discover its importance and requirements through this resource.
[^6]: FDA 21 CFR Part 820 outlines critical regulations for medical device safety. Learn more about its implications for manufacturers here.
[^7]: Learn about the key medical PCB applications that are crucial for modern healthcare, highlighting their importance in diagnostics and treatment.
[^8]: Explore the latest advancements in diagnostic imaging to understand how technology is transforming patient care and outcomes.
[^9]: Discover how PCB miniaturization is revolutionizing medical technology, leading to smaller, more efficient devices that enhance patient care.